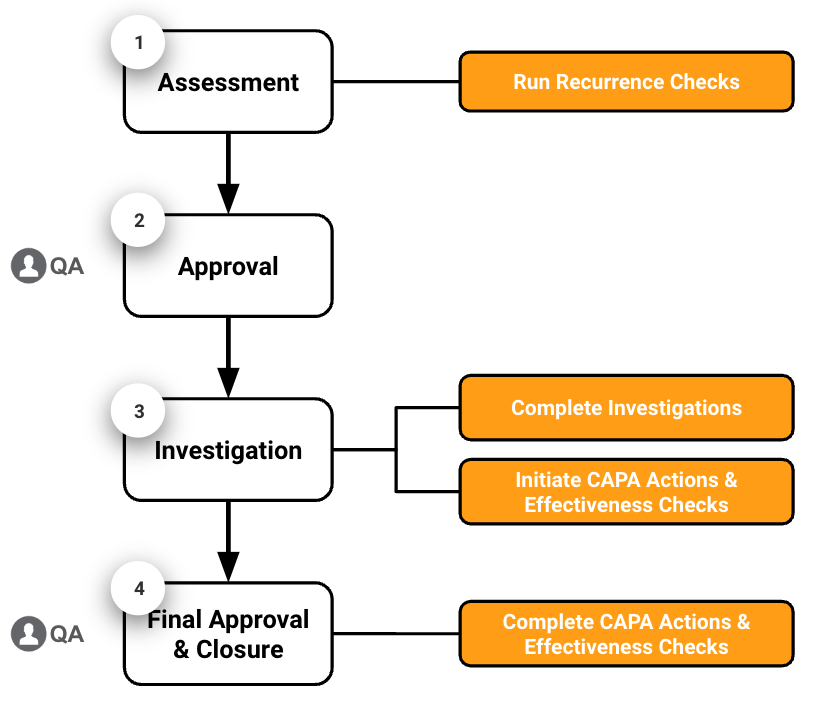
When a Deviation is determined to have a Rating of Major or Critical, the Deviation record is managed in four (4) main stages: assessment, approval, investigation, and final approval & closure.
- Assessment: The Deviation Owner reviews the Deviation record, runs a Recurrence Check to determine if the deviation has occurred before, and creates Extension Requests as needed.
- Approval: The Deviation Owner sends the Deviation to the QA Approver for approval. The QA Approver reviews and approves the Deviation.
- Investigation: The Deviation Owner determines the root cause, completes the Impact & Risk Analysis, completes the Investigation, creates CAPA Actions, creates Effectiveness Checks, and creates Extension Requests as needed.
- Final Approval & Closure: The QA Approver reviews the Deviation details and related records and approves the Deviation, which closes it. The CAPA Actions and Effectiveness Checks Owners complete their respective actions and checks.
Assessing Major and Critical Deviations
When a Deviation is created, Vault assigns a Complete Assessment task to the Deviation Owner. To complete the task, review the Deviation details, select the Rating, and document any other information as needed.
Complete the following steps to assess a Major or Critical Deviation:
- Access the Deviation from the assigned task. Vault opens the Deviation and displays a task banner with the task due date and instructions.
- Expand the Details section and click Edit () on the action bar.
- In the Details section, review and adjust any of the deviation details as needed.
- Select Major or Critical for the Rating.
- Click Save to save the Rating.
- Click Complete in the Complete Assessment task banner to open the Complete Assessment dialog and select the appropriate verdict:
- To send the Deviation for approval, select the Complete verdict and click Complete. Vault updates the Deviation status to In Approval and assigns a Quality Approval task to the Deviation QA Approver.
- To request cancelation of the Deviation, select the Request Cancelation verdict, enter the Cancelation Reason, and click Complete. Vault changes the Deviation status to Pending Cancelation and assigns an Approve Deviation Cancelation task to the QA Approver.
Investigating Major and Critical Deviations
When a Major or Critical Deviation receives its initial QA approval, Vault assigns the Deviation Owner a Complete Investigation & CAPA Plan task. To complete the task, document the required information regarding the Deviation investigation.
Complete the following steps to document Deviation investigation details:
- Access the Deviation from the assigned task. Vault opens the Deviation and displays a task banner with the task due date and instructions.
- Expand the Details section and click Edit () on the action bar.
- In the Details section, review and adjust the Rating if needed.
- Expand the Impact & Risk Analysis section and enter details regarding the impact of the Deviation and the risk analysis performed.
- Expand the Investigation Summary & Conclusion section and enter the results of the investigation.
- Expand the Plan Details section.
- For the CAPA Required field, select whether a CAPA Action is required:
- Select Yes if a CAPA Action must be completed as a result of the Deviation. The CAPA Actions section is displayed and at least one CAPA Action must be created before the Deviation investigation can be approved.
- Select No if a CAPA Action is not required. In the Rationale for No CAPA field, enter the justification for why a CAPA Action is not required.
- For the Effectiveness Check Required field, select whether an Effectiveness Check is required:
- Select Yes if an Effectiveness Check must be completed as a result of the Deviation. The Effectiveness Checks section is displayed and at least one Effectiveness Check must be created before the Deviation investigation can be approved.
- Select No if an Effectiveness Check is not required. In the Rationale for No Effectiveness Check field, enter the justification for why an Effectiveness Check is not required.
- Click Save to save the Deviation information.
Creating Investigations
At least one Investigation is required to be created and completed for Major and Critical Deviations.
Complete the following steps to create an Investigation:
- Expand the Investigations section and click Create. The Create Investigation page is displayed.
- Enter the Title of the Investigation.
- Enter a detailed Description of the Investigation.
- Select the Investigation Category.
- Select the Owning Department responsible for conducting the deviation.
- Click Save. Vault assigns the Investigation a Record Number, creates the Investigation record with a status of Define Team, and opens the Investigation page.
- Expand the Team section and click Manage Team.
- Select the Owner responsible for conducting the investigation.
- Click Save to save the selected Owner. Vault updates the Investigation status to In Investigation and assigns a Complete Investigation task to the Investigation Owner.
Completing Investigations
When the Deviation Owner creates an Investigation, Vault assigns the Investigation Owner a Complete Investigation task. To complete the task, conduct the investigation and document the results.
If you need additional time to complete an Investigation, create an Extension Request to request a later due date.
Complete the following steps to complete an Investigation:
- Access the Investigation from the assigned task. Vault opens the Investigation and displays a task banner with the task due date and instructions.
- Expand the Results section and click Edit () on the action bar.
- In the Results section, enter the results of the investigation.
- Click Save to save the results.
- Optional: Add Library References or Attachments as needed.
- Click Complete in the Complete Investigation task banner to open the Complete Investigation dialog.
- Click Complete in the dialog. Vault updates the Investigation status to Investigation Completed.
Creating CAPA Actions
CAPAs allow you to track actions taken to either correct or prevent issues found during the Deviation investigation.
If a Major or Critical Deviation’s CAPA Required field is set to Yes, at least one CAPA Action must be created and in the Initiated state or later before the Deviation can be sent for final approval.
Create CAPA Actions as needed during the Deviation investigation.
Creating Effectiveness Checks
Effectiveness Checks allow you to monitor the effectiveness of the change after it is implemented. Effectiveness Checks are created during the Deviation investigation and completed after the Deviation is closed.
If a Deviation’s Effectiveness Check Required field is set to Yes, at least one Effectiveness Check must be created and in the Initiated state before the Deviation can be sent for final approval.
Create Effectiveness Checks as needed during the Deviation investigation. Complete Effectiveness Check tasks are not assigned to the Effectiveness Check Owner until after the Deviation is closed and the Effectiveness Check Start Date has passed.
Adding Related Records & Resources
Add the following optional related records and resources as needed during the Deviation investigation process:
- Related Events: Allow you to link the Deviation to other Deviations or Change Controls.
- Library References: Allow you to create references to documents in the Library.
- Attachments: Allow you to attach files to the Deviation.
Creating Extension Requests
The due dates for Deviations, Investigations, CAPA Actions, and Effectiveness Checks cannot be changed after they are created. Once a Deviation is in the In Assessment status or later, you must create an Extension Request to adjust the due date for any of the above records.
Create Extension Requests as needed for Deviations, Investigations, CAPA Actions, and Effectiveness Checks during the investigation process. QA Approvers are required to approve Extension Requests before the associated due dates are changed. If an Extension Request is rejected and returned for revision, you can update the Extension Request and resubmit it.
Closing Major and Critical Deviations
Once the Deviation Owner completes the investigation and the appropriate details and records are documented, the Owner can send the Deviation to the QA Approver for final approval. After the QA Approver completes the final QA approval, the Deviation is automatically closed.
If any CAPA Actions or Effectiveness Checks are required, they are completed by their respective Owners after the Deviation is closed.
Complete the following steps to initiate final approval and closure for a Major or Critical Deviation:
- Access the Deviation from the assigned task. Vault opens the Deviation and displays a task banner with the task due date and instructions.
- Click Complete in the Complete Investigation & CAPA Plan task banner to open the Complete Investigation & CAPA Plan dialog.
- Click Complete in the dialog. Vault performs the following actions and status changes:
- The Deviation status is updated to In Final Approval.
- A Complete Final Approval task is assigned to the Deviation QA Approver.
- The status of CAPA Actions and Effectiveness Checks are updated to Pending Review.