Quality Basics: QMS allows you to create Change Control records to help you track and manage changes to your site’s controlled processes. The Change Control process allows for documenting desired changes, assessing their impact, and capturing the actions required for change implementation.
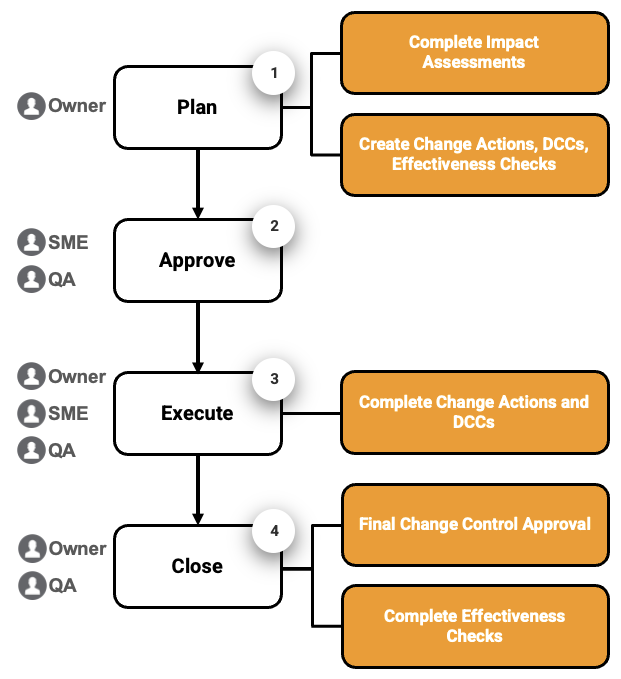
Quality Basics: QMS provides the tools you need to manage Change Controls in four (4) main stages: plan creation, approval, execution, and closure.
- Change Control Creation: Develop the change plan by creating Change Actions and Effectiveness Checks and completing Impact Assessments.
- Change Control Approval: Finalize the change plan and send it through subject matter expert (SME) approval and QA approval.
- Change Control Execution: Implement the change plan by completing all Change Actions and Document Change Controls (DCCs). If needed, create an Extension Request with QA approval to update the Change Control due date.
- Change Control Closure: Close the Change Control with final QA approval once all Change Actions and DCCs are completed. Complete Effectiveness Checks to assess the results of the change.
About Change Control Teams
When you create a Change Control, you are prompted to select the team members responsible for the Change Control. Records created from a Change Control, such as Impact Assessments, Change Actions, and Effectiveness Checks, also require you to select the responsible team members.
When you first create a record that requires a team, a team required icon () is displayed in the left panel and the Team section heading. After adding and saving the team members, the icon is removed and the record can progress to its next lifecycle stage.
The following team roles are available, but not all roles are required for all records:
- Owner: The Owner assigned to a record is responsible for managing and performing the main actions or tasks associated with the record. For example, the Owner of a Change Control is responsible for creating the change plan, including the impact and risk analysis, and creating the Change Actions required to implement the change.
- Approver: The Approver assigned to a record is responsible for reviewing the information documented in the record and either approving or rejecting the record at their discretion. The Approver can be a subject matter expert, quality expert, or other user. For example, the Approver of a Change Action is responsible for confirming that the action associated with the change was performed according to the Actions Taken information documented in the Change Action record.
- QA Approver: The QA Approver assigned to a record is responsible for performing the final quality review and approval. The QA Approver is typically a member of the Quality team. For example, the QA Approver is responsible for confirming that the outcome details in an Effectiveness Check were performed according to the Results information documented in the Effectiveness Check record.
When defining a team, you can select multiple Approvers and QA Approvers. If you select multiple users for these roles, all selected users must select the Approve verdict for the record to be considered approved. If at least one approver selects the Reject verdict, the record is rejected and returned to the Owner.